QUANTUM NUMBERS
Hey guys,my name is Chaithanya.Hoping that you are all fine.this blog is about the 'Quntum Numbers'.I hope you can learn about the quantum numbers by this blog.So,let's get started.
Quantum Numbers
Each electron in an atom is described by a set of three numbers n,l,m. These numbers indicate the probability of finding the electron in space around the nucleus.
These Quantum numbers describe the space around the nucleus where the electrons are found and also their energies.These are called Atomic Orbitals.
1.Principle Quantum Numbers:
- It is related to Size and Energy of the main shell.
- It is denoted by 'n'.
- 'n' has positive integer values of 1,2,3.......
- As 'n' increases , the shells become larger and the electrons in those shells are farther from the nucleus.
Shell K L M N
n 1 2 3 4
2.Angular-Momentum Quantum Numbers: (l)
- The Angular-Momentum Quantum number 'l' has integer values from 0 to n-1.
- Each 'l' value represents one sub-shell.
- Each value of 'l' is related to the shape of a particular sub-shell in space around the nucleus.
'l' 0 1 2 3
Name of the sub-shell s p d f
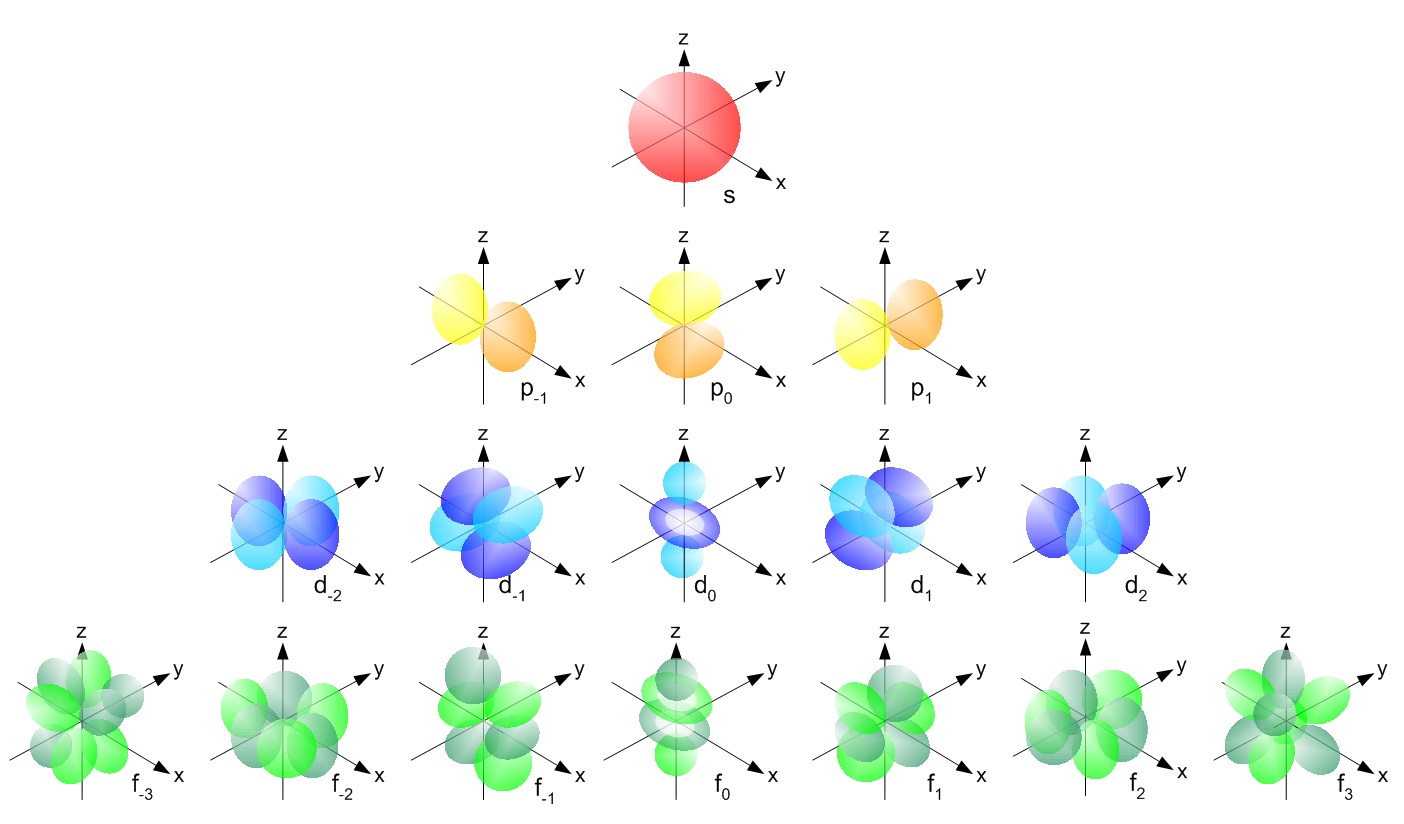
3.Magnetic Quantum Number:
- It has the integer values between -l and l,including '0'.
- For a certain value of 'l' ,there are (2l+1) integer values of m.
S orbital - Spherical shape ; p-orbial -Dumbell shape ; D orbital: double dumbell shape .
4.Spin Quantum Number(ms):
- This quantum number refers to two possible orientations of the soin of an electron , one clockwise and the other anti-clockwise direction spin.
- This is denoted by ms.
- This is represented by +1/2 and -1/2.
- If both are positive values, then the spins are parallel, otherwise the spins are anti-parallel.


Comments
Post a Comment